Draw the product of an SN2 reaction shown below is a topic of great significance in organic chemistry. Understanding the product formation in SN2 reactions is crucial for comprehending the fundamental principles governing nucleophilic substitution mechanisms. This guide delves into the intricacies of SN2 reactions, providing a thorough examination of the product formation, stereochemistry, and factors influencing the reaction rate.
SN2 reactions, characterized by their bimolecular nature, involve the substitution of a nucleophile for a leaving group at a saturated carbon atom. The reaction proceeds through a concerted mechanism, with the nucleophile attacking the electrophilic carbon simultaneously as the leaving group departs.
This concerted mechanism results in inversion of the stereochemistry at the reaction center, a defining characteristic of SN2 reactions.
Draw the Product
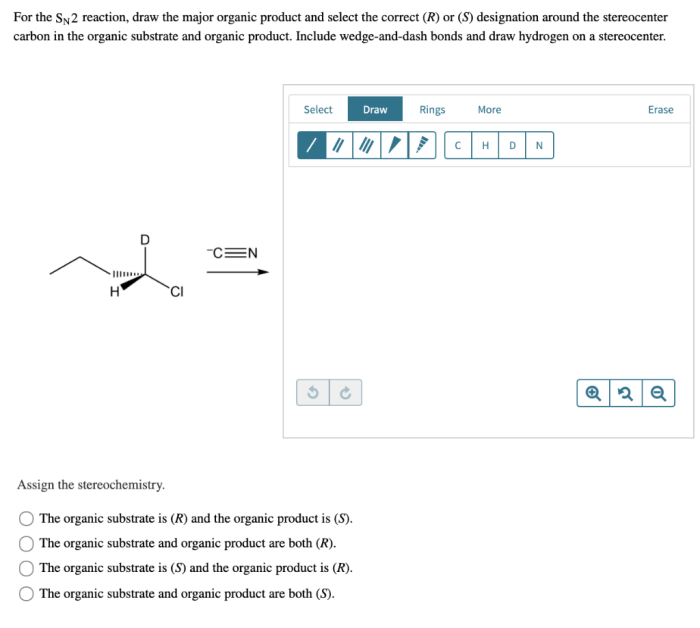
To draw the product of an SN2 reaction, first identify the nucleophile and the electrophile. The nucleophile is the species that attacks the electrophile, and the electrophile is the species that is attacked by the nucleophile.
Once the nucleophile and electrophile have been identified, the product can be drawn by following these steps:
- The nucleophile attacks the electrophile, forming a new bond between the nucleophile and the carbon atom that was previously bonded to the electrophile.
- The leaving group (the group that was originally bonded to the carbon atom that was attacked by the nucleophile) is expelled.
- The product is the molecule that is formed by the combination of the nucleophile and the electrophile, minus the leaving group.
The stereochemistry of the product is determined by the orientation of the nucleophile when it attacks the electrophile. If the nucleophile attacks from the same side of the molecule as the leaving group, the product will have an inverted stereochemistry.
If the nucleophile attacks from the opposite side of the molecule as the leaving group, the product will have a retained stereochemistry.
Reaction Mechanism
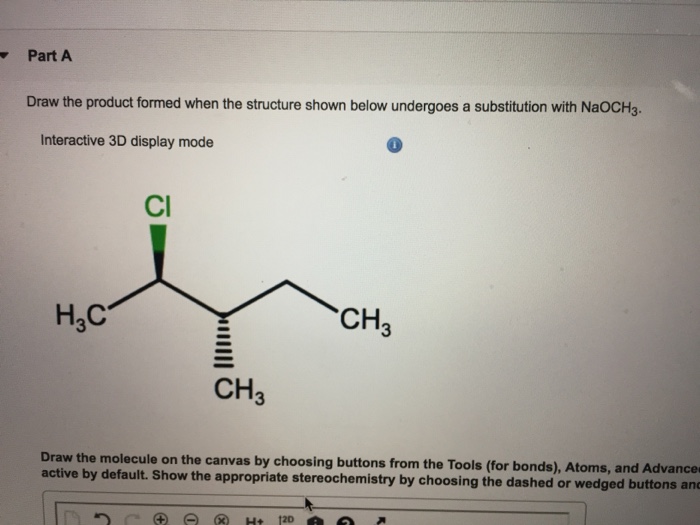
The SN2 reaction is a one-step reaction that occurs in a single concerted step. The nucleophile attacks the electrophile, and the leaving group is expelled simultaneously.
The rate of the SN2 reaction is determined by the following factors:
- The nucleophilicity of the nucleophile
- The electrophilicity of the electrophile
- The solvent
The nucleophilicity of a nucleophile is a measure of its ability to donate electrons to an electrophile. The electrophilicity of an electrophile is a measure of its ability to accept electrons from a nucleophile. The solvent can affect the rate of the SN2 reaction by solvating the nucleophile or the electrophile, or by changing the polarity of the reaction medium.
Factors Affecting the Reaction
The rate of the SN2 reaction is affected by the following factors:
- The nucleophilicity of the nucleophile
- The electrophilicity of the electrophile
- The solvent
- The temperature
- The concentration of the reactants
The nucleophilicity of the nucleophile is a measure of its ability to donate electrons to an electrophile. The electrophilicity of the electrophile is a measure of its ability to accept electrons from a nucleophile. The solvent can affect the rate of the SN2 reaction by solvating the nucleophile or the electrophile, or by changing the polarity of the reaction medium.
The temperature can affect the rate of the SN2 reaction by increasing the kinetic energy of the reactants. The concentration of the reactants can affect the rate of the SN2 reaction by increasing the number of collisions between the nucleophile and the electrophile.
Examples of SN2 Reactions: Draw The Product Of An Sn2 Reaction Shown Below
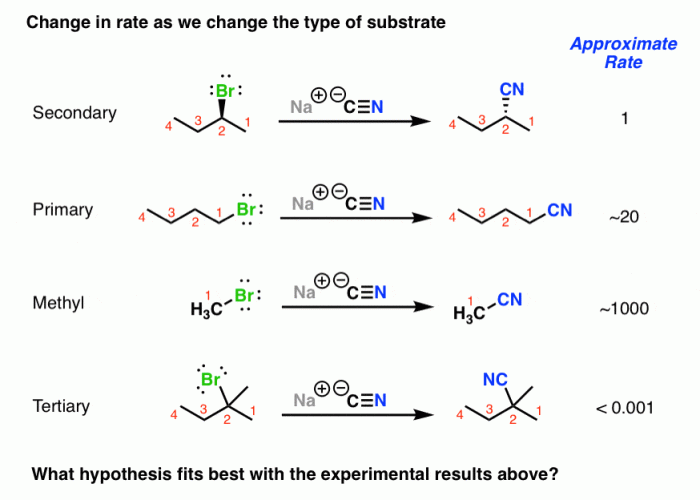
SN2 reactions are a common type of organic reaction. Some examples of SN2 reactions include:
- The reaction of a hydroxide ion with an alkyl halide
- The reaction of an alkoxide ion with an alkyl halide
- The reaction of an amine with an alkyl halide
The following table shows some examples of SN2 reactions, along with the reactants, products, and stereochemistry of the products:
| Reactants | Products | Stereochemistry |
|---|---|---|
| NaOH + CH3Br | CH3OH + NaBr | Inverted |
| NaOCH3 + CH3Br | CH3OCH3 + NaBr | Inverted |
| NH3 + CH3Br | CH3NH2 + HBr | Inverted |
Applications of SN2 Reactions
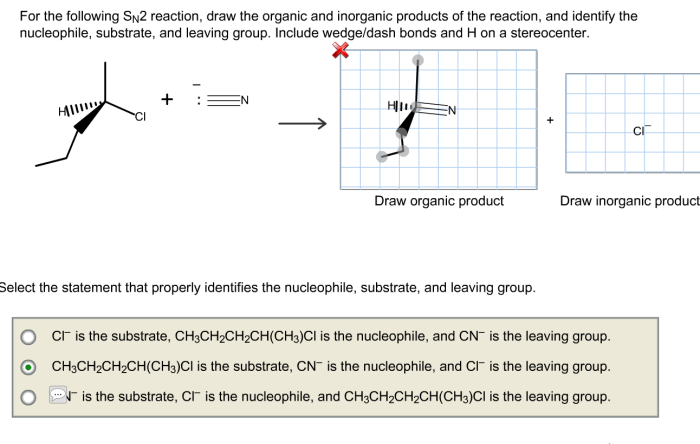
SN2 reactions are used in a variety of organic synthesis applications. Some examples of the applications of SN2 reactions include:
- The synthesis of ethers
- The synthesis of esters
- The synthesis of amines
SN2 reactions are also used in a variety of industrial processes, such as the production of plastics and pharmaceuticals.
Essential Questionnaire
What is the key feature of an SN2 reaction?
The key feature of an SN2 reaction is its concerted, bimolecular mechanism, where the nucleophile attacks the electrophilic carbon simultaneously as the leaving group departs, resulting in inversion of stereochemistry.
What factors influence the rate of an SN2 reaction?
The rate of an SN2 reaction is influenced by the nucleophilicity of the nucleophile, the electrophilicity of the electrophile, and the solvent effects, such as polarity and protic/aprotic nature.
How can SN2 reactions be used in organic synthesis?
SN2 reactions are valuable in organic synthesis for the selective formation of carbon-carbon bonds and the construction of complex organic molecules, such as pharmaceuticals and natural products.