Draw the lewis structure of methyl mercaptan – Delving into the intricacies of chemical structures, this discourse on drawing the Lewis structure of methyl mercaptan unravels a fascinating journey into the realm of molecular representation. Understanding Lewis structures holds immense significance, providing a visual depiction of the arrangement of atoms and their chemical bonds, thereby elucidating the molecular geometry and properties that govern their behavior.
As we embark on this exploration, we shall meticulously identify the constituent atoms of methyl mercaptan, unravel the intricacies of their bonding patterns, and construct its Lewis structure with precision. Along the way, we will encounter the concept of resonance structures and delve into their implications for the overall molecular structure and bonding.
Finally, we will ascertain the molecular geometry of methyl mercaptan based on its Lewis structure, uncovering its polarity and other defining characteristics.
Draw the Lewis Structure of Methyl Mercaptan
In chemistry, understanding Lewis structures is crucial for visualizing and comprehending the bonding and electronic configuration of molecules. A Lewis structure represents the arrangement of atoms and their valence electrons, providing insights into molecular geometry, bonding, and properties.
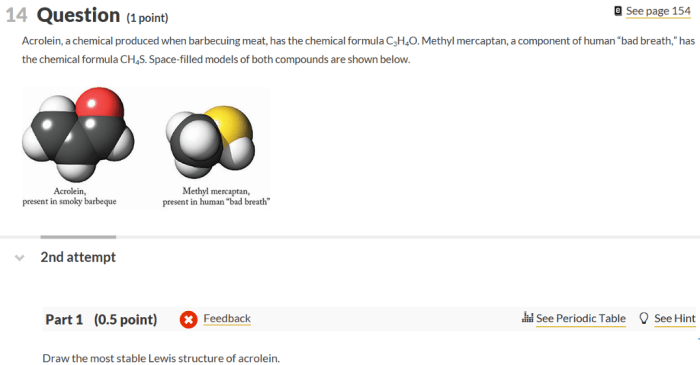
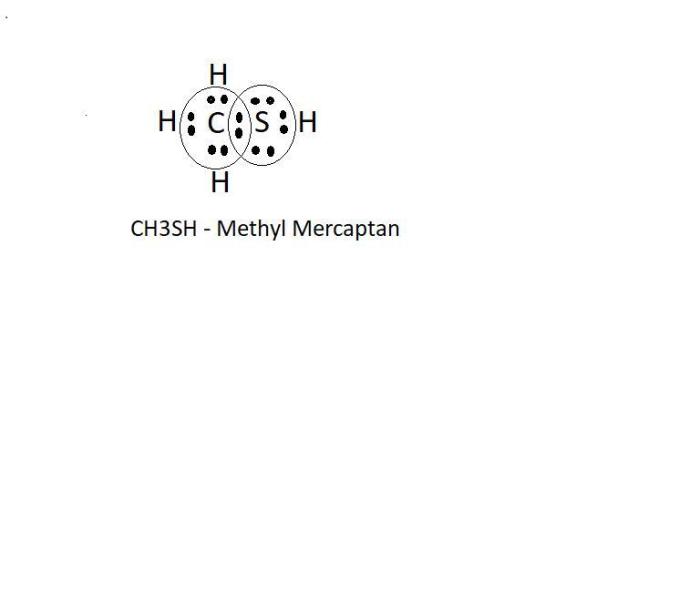
Methyl mercaptan, also known as methanethiol, is an organic compound with the chemical formula CH 3SH. Drawing the Lewis structure of methyl mercaptan allows us to determine its molecular structure and predict its properties.
Identifying Atoms and their Connectivity
Methyl mercaptan consists of four atoms: one carbon (C), three hydrogen (H), and one sulfur (S). The carbon atom is the central atom, bonded to the three hydrogen atoms by single bonds and to the sulfur atom by a single bond.
Drawing the Lewis Structure, Draw the lewis structure of methyl mercaptan
To draw the Lewis structure of methyl mercaptan, we follow these steps:
- Determine the total number of valence electrons: Carbon has 4, each hydrogen has 1, and sulfur has 6, giving a total of 12 valence electrons.
- Connect the atoms with single bonds, using two valence electrons for each bond. This gives us a structure with C-H, C-H, C-H, and C-S bonds.
- Distribute the remaining valence electrons as lone pairs on the atoms. In this case, the sulfur atom has two lone pairs, and each hydrogen atom has one lone pair.
Resonance Structures
Methyl mercaptan does not exhibit resonance structures because there are no equivalent resonance structures that contribute to the overall structure.
Molecular Geometry and Properties
Based on its Lewis structure, methyl mercaptan has a tetrahedral molecular geometry around the carbon atom. The C-H bonds are polar, with a partial positive charge on the hydrogen atoms and a partial negative charge on the carbon atom. The C-S bond is also polar, with a partial positive charge on the carbon atom and a partial negative charge on the sulfur atom.
These polar bonds result in a net dipole moment for the molecule.
Frequently Asked Questions: Draw The Lewis Structure Of Methyl Mercaptan
What is the hybridization of the carbon atom in methyl mercaptan?
sp 3
How many lone pairs of electrons are present on the sulfur atom in methyl mercaptan?
Two
What is the molecular shape of methyl mercaptan?
Tetrahedral